Republished with permission from The Conversation, by Arindam Sau, University of Colorado Boulder; Mihai Popescu, Colorado State University, and Xin Liu, Colorado State University
Perfluoroalkyl and polyfluoroalkyl substances, or PFAS, have earned the nickname of forever chemicals from their extraordinary ability to stick around in the environment long after they’ve been used.
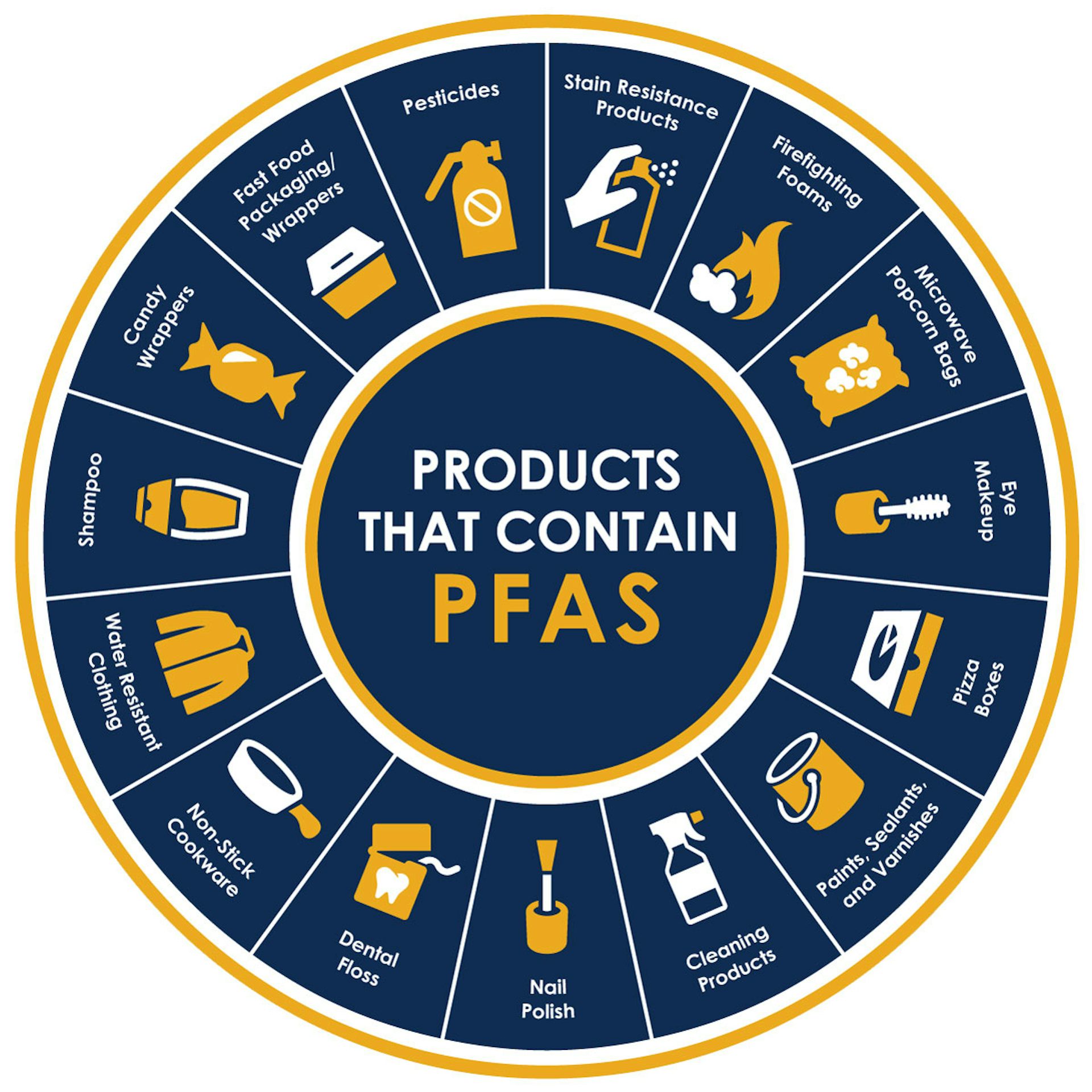
These synthetic compounds, commonly used in consumer products and industrial applications for their water- and grease-resistant properties, are now found practically everywhere in the environment.
While many chemicals will degrade relatively quickly after they’re disposed of, PFAS can stick around for up to 1,000 years. This durability is great for their use in firefighting foams, nonstick cookware, waterproof clothing and even food packaging.
However, their resilience means that they persist in soil, water and even living organisms. They can accumulate over time and affect the health of both ecosystems and humans.
Some initial research has shown potential links between PFAS exposure and various health issues—including cancers, immune system suppression and hormone disruption. These concerns have led scientists to search for effective ways to break down these stubborn chemicals.
We’re a team of researchers who developed a chemical system that uses light to break down bonds between carbon and fluorine atoms. These strong chemical bonds help PFAS resist degradation. We published this work in Nature in November 2024, and we hope this technique could help address the widespread contamination these substances cause.
Why PFAS Compounds Are So Hard to Break Down
PFAS compounds have carbon-fluorine bonds, one of the strongest in chemistry. These bonds make PFAS incredibly stable. They resist the degradation processes that usually break down industrial chemicals—including hydrolysis, oxidation and microbial breakdown.
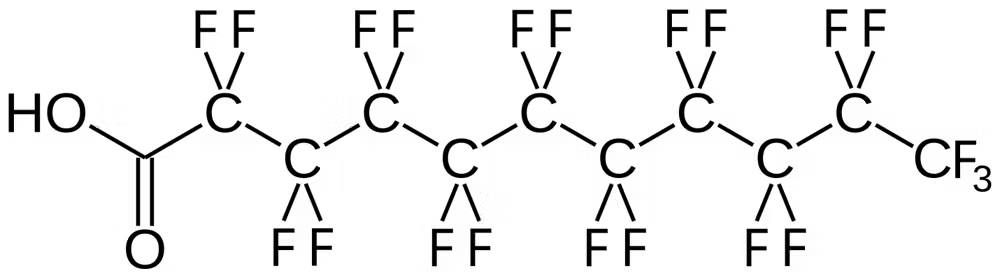
The carbon-fluorine bonds in PFAS, like this one, perfluoroundecanoic acid, make the molecules very stable. Bert.Kilanowski/Wikimedia Commons
Conventional water treatment methods can remove PFAS from water, but these processes merely concentrate the contaminants instead of destroying them. The resulting PFAS-laden materials are typically sent to landfills. Once disposed of, they can still leach back into the environment.
The current methods for breaking carbon-fluorine bonds depend on use of metals and very high temperatures. For example, platinum metal can be used for this purpose. This dependence makes these methods expensive, energy-intensive and challenging to use on a large scale.
How Our New Photocatalytic System Works
The new method our team has developed uses a purely organic photocatalyst. A photocatalyst is a substance that speeds up a chemical reaction using light, without being consumed in the process. Our system harnesses energy from cheap blue LEDs to drive a set of chemical reactions.
After absorbing light, the photocatalyst transfers electrons to the molecules containing fluorine, which breaks down the sturdy carbon-fluorine bonds.
By directly targeting and dismantling the molecular structure of PFAS, photocatalytic systems like ours hold the potential for complete mineralization. Complete mineralization is a process that transforms these harmful chemicals into harmless end products, like hydrocarbons and fluoride ions, which degrade easily in the environment. The degraded products can then be safely reabsorbed by plants.

Photocatalysis refers to accelerating a reaction by using light particles, called photons. Miyake Group
Potential Applications and Benefits
One of the most promising aspects of this new photocatalytic system is its simplicity. The setup is essentially a small vial illuminated by two LEDs, with two small fans added to keep it cool during the process. It operates under mild conditions and does not use any metals, which are often hazardous to handle and can sometimes be explosive.
The system’s reliance on light—a readily available and renewable energy source—could make it economically viable and sustainable. As we refine it, we hope that it could one day operate with minimal energy input, outside of the energy powering the light.
This platform can also transform other organic molecules that contain carbon-fluorine bonds into valuable chemicals. For instance, thousands of fluoroarenes are commonly available as industrial chemicals and laboratory reagents. These can be transformed into building blocks for making a variety of other materials, including medicines and everyday products.
Challenges and Future Directions
While this new system shows potential, challenges remain. Currently, we can degrade PFAS only on a small scale. While our experimental setup is effective, it will require substantial scaling up to tackle the PFAS problem on a larger level. Additionally, large molecules with hundreds of carbon-fluorine bonds, like Teflon, do not dissolve into the solvent we use for these reactions, even at high temperatures.
As a result, the system currently can’t break down these materials, and we need to conduct more research.
We also want to improve the long-term stability of these catalysts. Right now, these organic photocatalysts degrade over time, especially when they’re under constant LED illumination. So, designing catalysts that retain their efficiency over the long term will be essential for practical, large-scale use. Developing methods to regenerate or recycle these catalysts without losing performance will also be key for scaling up this technology.
With our colleagues at the Center for Sustainable Photoredox Catalysis, we plan to keep working on light-driven catalysis, aiming to discover more light-driven reactions that solve practical problems. SuPRCat is a National Science Foundation-funded nonprofit Center for Chemical Innovation. The teams there are working to develop reactions for more sustainable chemical manufacturing.
The end goal is to create a system that can remove PFAS contaminants from drinking water at purification plants, but that’s still a long way off. We’d also like to one day use this technology to clean up PFAS-contaminated soils, making them safe for farming and restoring their role in the environment.![]()
Arindam Sau, Ph.D. Candidate in Chemistry, University of Colorado Boulder; Mihai Popescu, Postdoctoral Associate in Chemistry, Colorado State University, and Xin Liu, Postdoctoral Scholar in Chemistry, Colorado State University
This article is republished from The Conversation under a Creative Commons license. Read the original article.

The Conversation
The Conversation is a nonprofit, independent news organization dedicated to unlocking the knowledge of experts for the public good. We publish trustworthy and informative articles written by academic experts for the general public and edited by our team of journalists.